The sum of the percentages of the specific isotopes must add up to 100. Mass of isotope abundance 36 96590 24 47 34 96885 75 53 atoms and isotopes worksheet.

How To Find The Natural Abundance Of An Isotope
Then calculate the average atomic mass of lead.
. The mass of antimony-121 is 120904 amu and the mass of antimony-123 is 122904 amu. The four isotopes oflead are shown below each with its percent by mass abundance and the composition of its nucleus. 3 1 9 6.
12 24Mg Percent abundance. Atomic mass mass 1 1 mass 2 2. 1 silicon-28 Percent abundance - 9223 Atomic mass - 2797693 amu M1 mass x percent abundance 100 2797693 amu x 9223 100 258031225 amu silicon-29 Percent abundance - 468 Atomic mass - 2897649 amu M2 mass x per.
Isotope Practice Worksheet 1. Calculating Average Atomic Mass Worksheet. 12 26Mg Percent abundance.
Model 1 that supports your answer. Chlorine has two naturally occurring isotopes 35 Cl 349689 amu and 37 Cl 369659 amu. Then calculate the mass numbers.
Using the following data calculate the average atomic mass of magnesium give your answer to the nearest 01 u. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l 0. Divide the mass of each isotope beans peas and corn by the number of each isotope to get the average mass of each isotope.
Divide the number of each isotope the total number of particles and multiply by 100 to get the percent abundance of each isotope. Weighted average of all the elements isotopes based on their natural abundance. How to Calculate Atomic Mass - ThoughtCo To calculate the average mass first convert the percentages into fractions divide them by 100.
Isotope information is provided below. 6 12C 6 13C. Boron has two naturally occurring isotopes.
Calculating Average Atomic Mass Worksheet. Determine both the percentage abundance and the relative isotopic mass of the second isotope. If the atomic masses of these isotopes are given calculating the percentage abundance of each of the isotopes is possible.
Average atomic massthe weighted average of the masses relative abundance of all the isotopes of an element. Calculating Average Atomic Mass The average atomic mass of an element is the sum of the masses of its isotopes each multiplied by its natural abundance the decimal associated with percent of atoms of that element that are of. Chlorine consists of two isotopes with masses of 35 abundance 75 and 37 abundance of 25.
If the abundance of 85 Rb is 722 and the abundance of 87 Rb is 278 what is the average atomic mass of rubidium. Write your final answer for Abundance in the Abundance column. For example atom percent 13C C12C 13C100 1 A closely related term is the fractional abundance fractional abundance of 13C 13F 13F 13C12C 13C 2.
Chlorine has two stable isotopes 3 5 C l and 3 7 C l with atomic masses 349689 u and 369659 u respectively. A dfrac b x c 100 - x 100 Here A Media Atomic Massesb Atomic mass of an isotopec atomic mass of the second isotope and x abundance percentage of the first isotopenote. Fill in the table with the correct information.
Isotope using the equation in the top row of the column. This value goes in the Mass Due to Isotope column. Calculate its average atomic mass.
The value of atomic mass of copper is 63546. Carbon 6 C 12011 isotope abundance mass amu. The abundance percentage of isotopes of an element is determined using the formula given.
C 12011 average mass isotope i x mass isotope isotope 2 mass 2. Isotope effects and their consequences in open and closed systems. Avg Mass mass mass.
This problem demonstrates finding the percent abundance of isotopes with known average atomic mass. Determine the percent abundance and isotopic mass of 26 Mg. 1 Three isotopes of Silicon occur in nature.
Using the following data first calculate the approximate atomic mass of each isotope. If chlorine has an average. Divide the percent abundance from Step 4 by 100 to get the relative abundance.
Antimony has two naturally occurring isotopes. 79Br of I r 7892 and percentage abundance 5054 and 81Br. 7 Calculate the.
Given below is a problem with its detailed solution explaining how to find the same. Write this number in the appropriate column. The isotopes generally have the same physical and chemical.
Relative abundance of isotopes worksheet learning objectives Define atomic weight Calculate atomic weight from percent abundance Manipulate the atomic. 82p 82p 122n 124n 82p 125n 82p 126n 137 2626 2082 5155 4. Multiply the Mass of the isotope by the Abundance.
In this worksheet we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Consider that an element has two isotopes. Isotope I r abundance 64Zn 6393 4889 66Zn 6593 2781 67Zn 6693 411 68Zn 6793 1857 70Zn 6993 062 6 Bromine has a relative atomic mass of 7991.
Find the percent abundances of 10 B and 11 B given the isotopic mass of 10 B 100129 amu and the isotopic mass of 11 B 110093 amu. Absolute abundances of isotopes are com-monly reported in terms of atom percent. Find the percentage.
The chlorine isotope with 18 neutrons has an Page 310. If their relative isotopic masses and percentage abundances are 3197 950 3297 077 and 3397 423 respectively. View the full answer.
Calculate the average atomic mass of bromine showing all work. Displaying top 8 worksheets found for abundance of isotopes. Using the average mass from the periodic table calculate the abundance of each isotope.
Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Here are three isotopes of an element. 12 24Mg Percent abundance.
Silicon-28 9223 2797693 amu Silicon-29 468 2897649 amu Silicon-30 309 2997377 amu Calculate the average atomic mass for the three isotopes of Silicon. Calculating atomic mass worksheet. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance.
12 25Mg Percent abundance. The relative abundance of an isotope means the percentage of that particular isotope that occurs in natureMost elements are made up of a mixture of isotopes. How to Calculate Percent Abundance.
The relative atomic mass is the weighted average of the isotopic masses. Naturally occurring boron B consists of two isotopes with a mass of 10 and 11. Be sure to use the function on your calculator or use the percent in decimal form.
Calculating Average Atomic mass. Relative abundance of isotope on spectrum sum of all relative isotope abundances on spectrum nitrogen-14 100 100 037 0996 or 996 nitrogen-15 037 100 037 0004 or 04. Calculate the relative atomic mass of strontium.
It has two isotopes.

Solving For Percent Abundance With Isotopes Chemistry Sample Problem Youtube
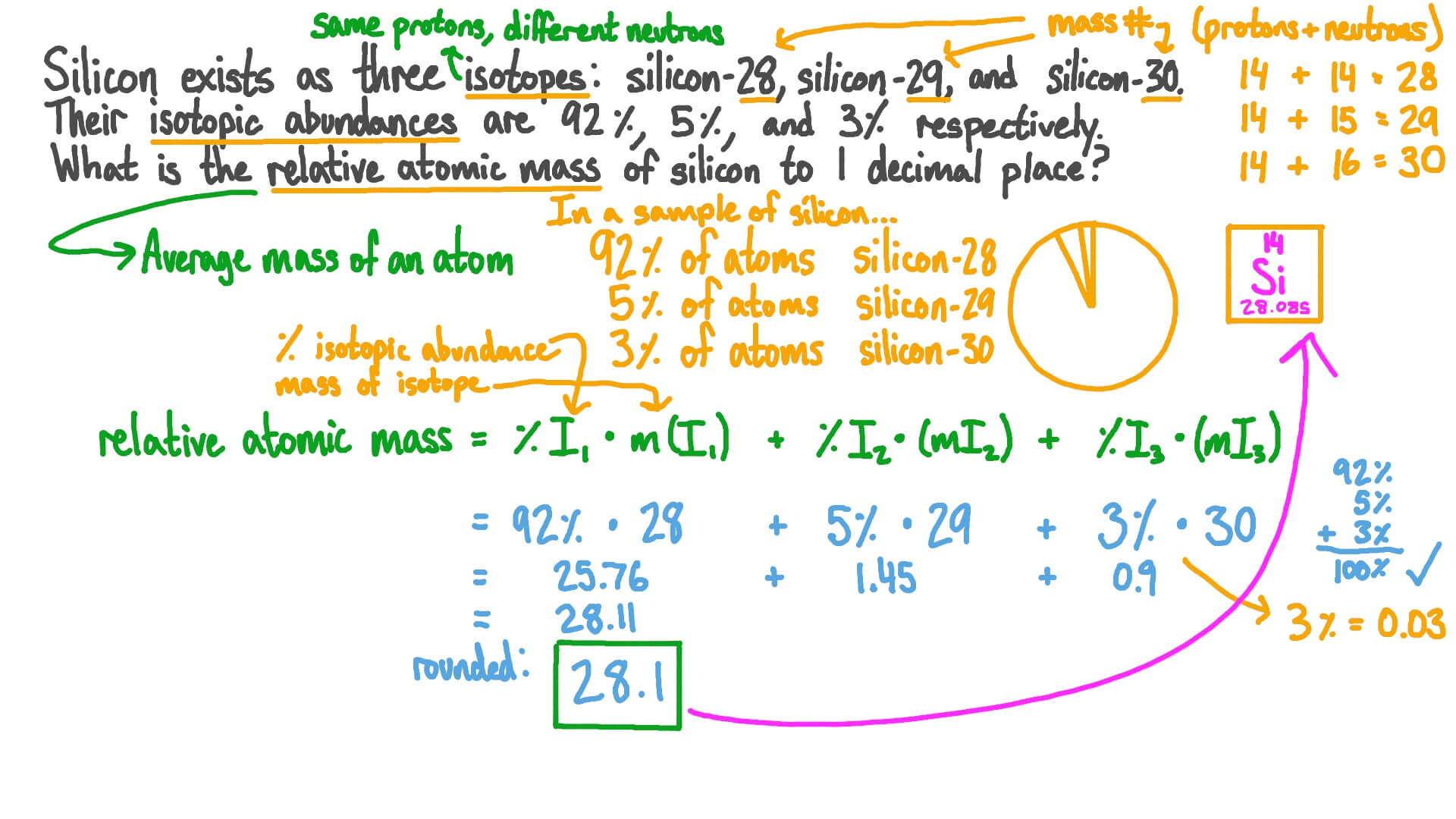
Question Video Using Relative Abundance Of Isotopes To Calculate Relative Atomic Mass Nagwa
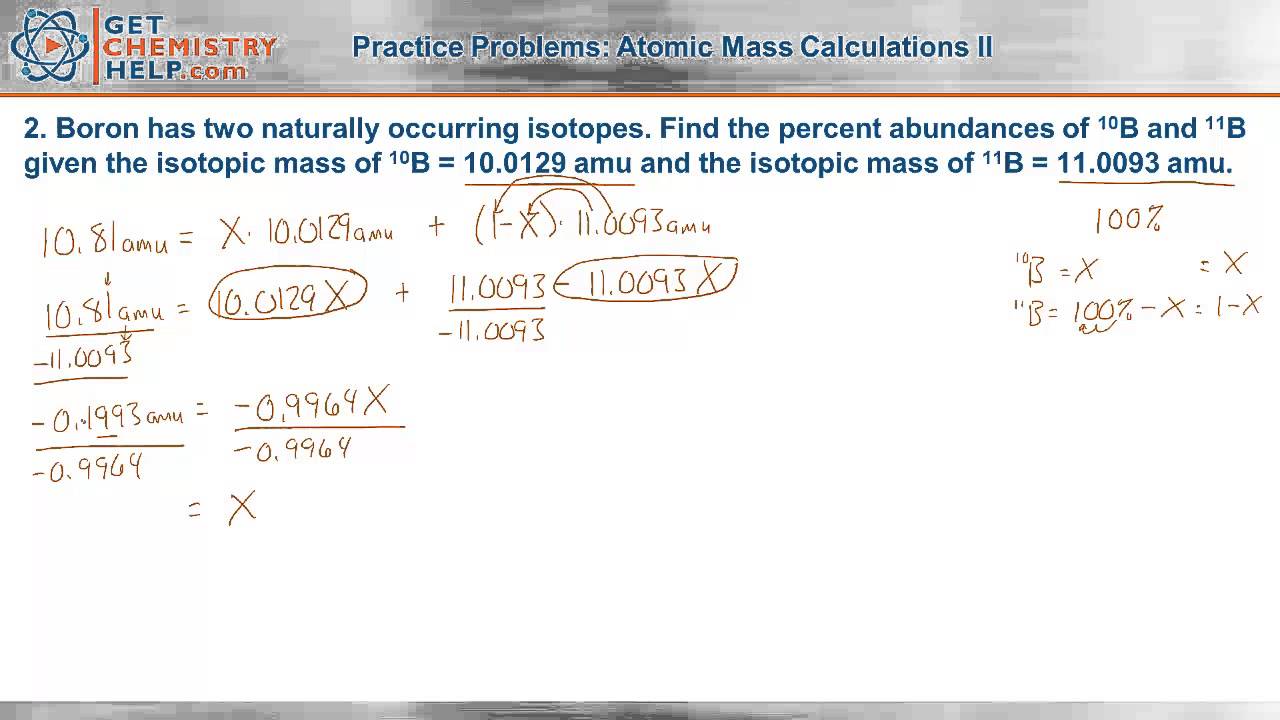
Chemistry Practice Problems Atomic Mass Calculations Ii Youtube

How To Calculate Percentage Abundance Using Atomic And Isotopic Masses

Question Video Calculating The Relative Atomic Mass Of Chlorine From Isotopic Abundances Nagwa
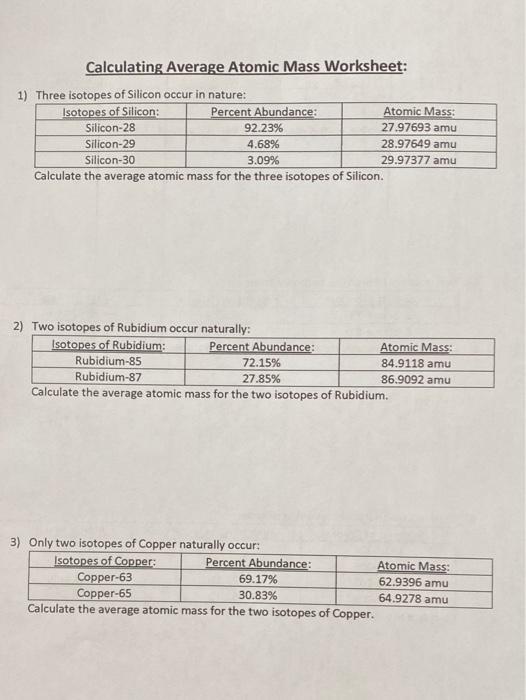
Solved Calculating Average Atomic Mass Worksheet 1 Three Chegg Com
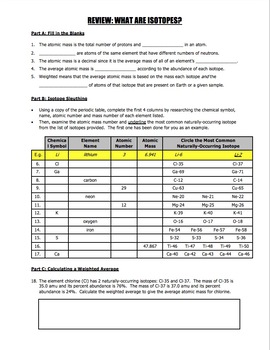
Worksheet Isotopes Percent Abundance And Weighted Averages Tpt
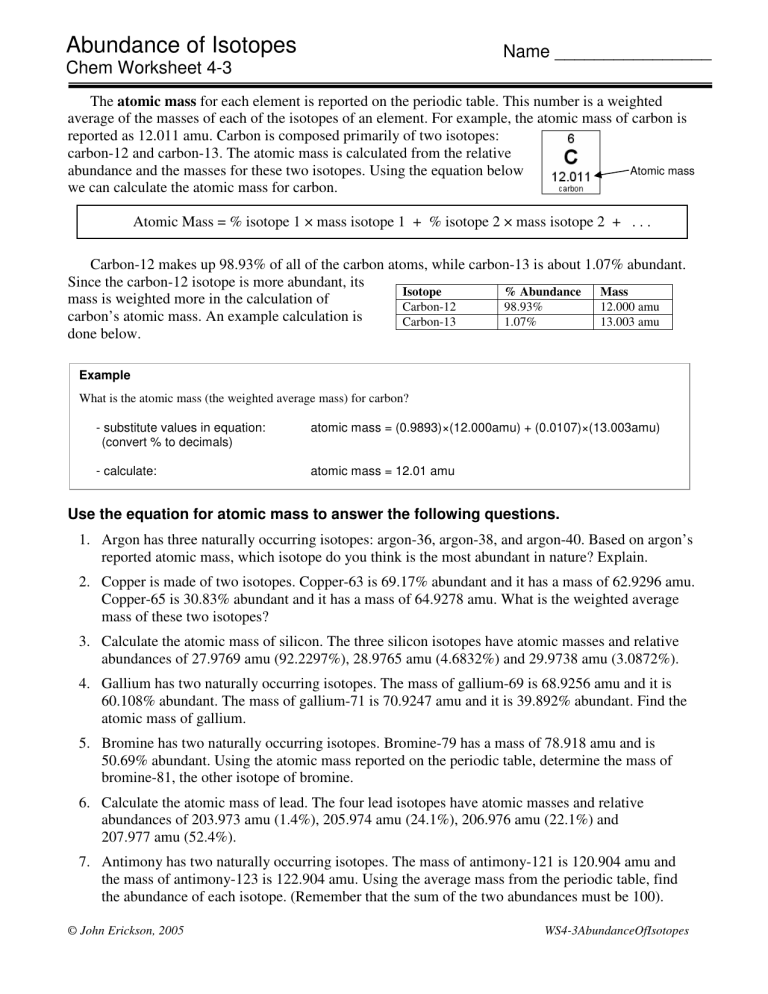
Abundance Of Isotopes Name Chem Worksheet 4 3

Average Atomic Mass And Percent Abundance Worksheet 2 And Key Pdf Isotope Chemical Elements
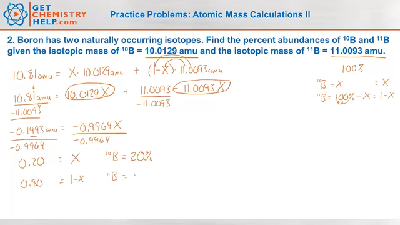
Chemistry Practice Problems Atomic Mass Calculations Ii Get Chemistry Help

Isotopes Relative Abundance Calculations Structured Worksheet Teaching Resources


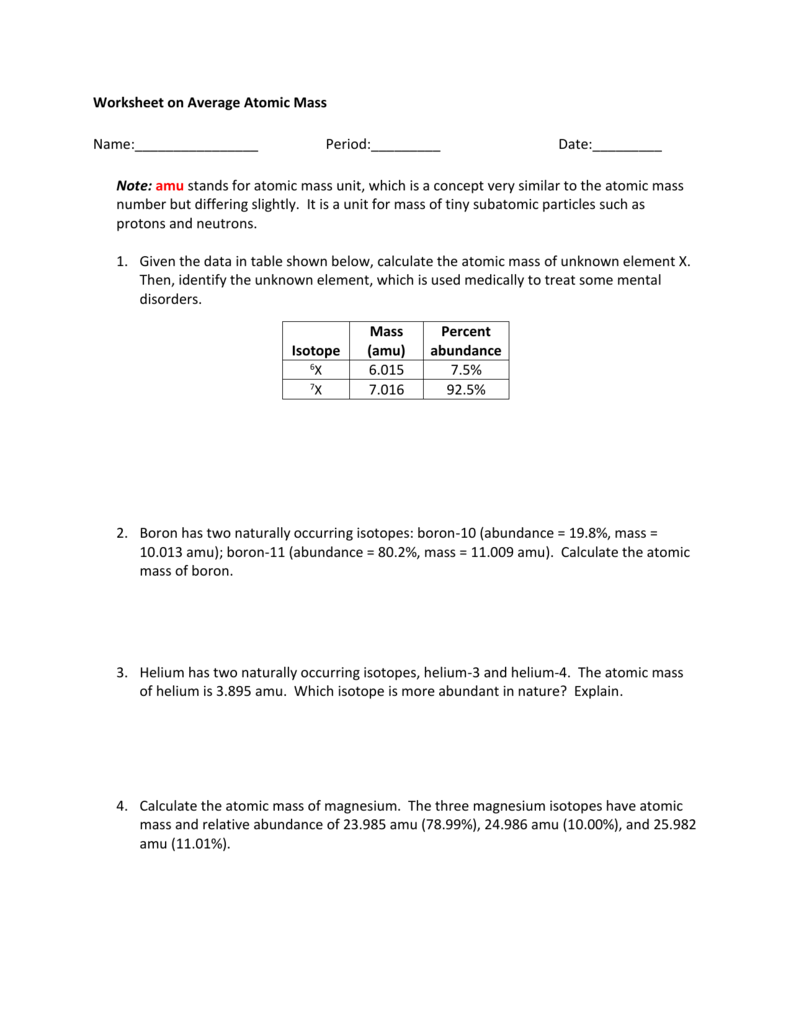
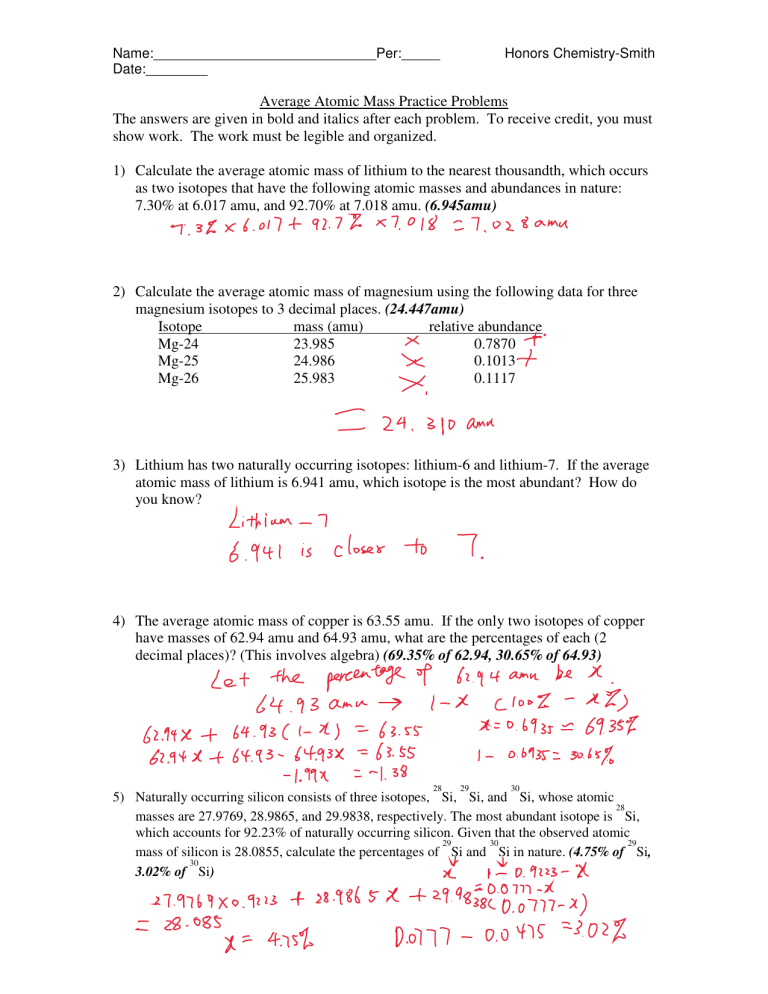


0 Komentar